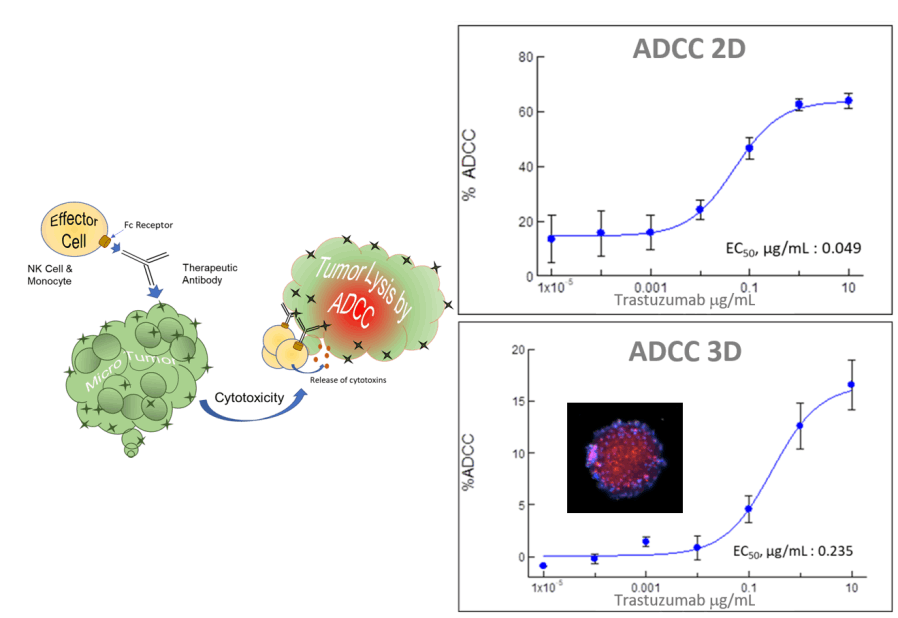
BIOENSIS Immuno-oncology Platform allows for better decision-making earlier in the drug development process. Our unique 2D/3D antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) platforms enable robust evaluation of therapeutic antibody efficacy. These platforms faithfully model critical physiological parameters such as cell–cell interactions, metabolic gradients, and antibody diffusion/distribution—providing data that more accurately predict in vivo performance.
Cell proliferation assays. Our proliferation assays provide a systematic approach to evaluate anti-cancer agents across a broad panel of cancer cell lines. By integrating efficacy and biomarker correlation studies, and using 3D cultures grown under physiologically relevant conditions, we help identify promising drug candidates with improved translational potential, minimizing the risk of costly late-stage failures.
BIOENSIS’ cell invasion/migration assays are designed to assess compound activity in key cancer processes such as tumor initiation, progression, metastasis, and tissue remodeling. These assays quantitatively measure a compound’s ability to inhibit invasive cancer and endothelial cell movement across basement membranes.
Immune-cell killing assays. These assays evaluate the capacity of therapeutic antibodies to activate T cells and NK cells to induce targeted cytotoxicity against tumor cells, providing insight into immune-mediated killing mechanisms.
Phagocytosis assays. Our ADCP assays assess the ability of monocyte-derived macrophages to eliminate tumor cells via antibody-mediated phagocytosis, offering a critical dimension in understanding immune effector function.
Please contact us to discuss your study requirements and an up to date list of available cell lines info@bioensis.com